SKU: Z-Path-High Risk HPV

Interested in this product?
Please contact us to place an order.
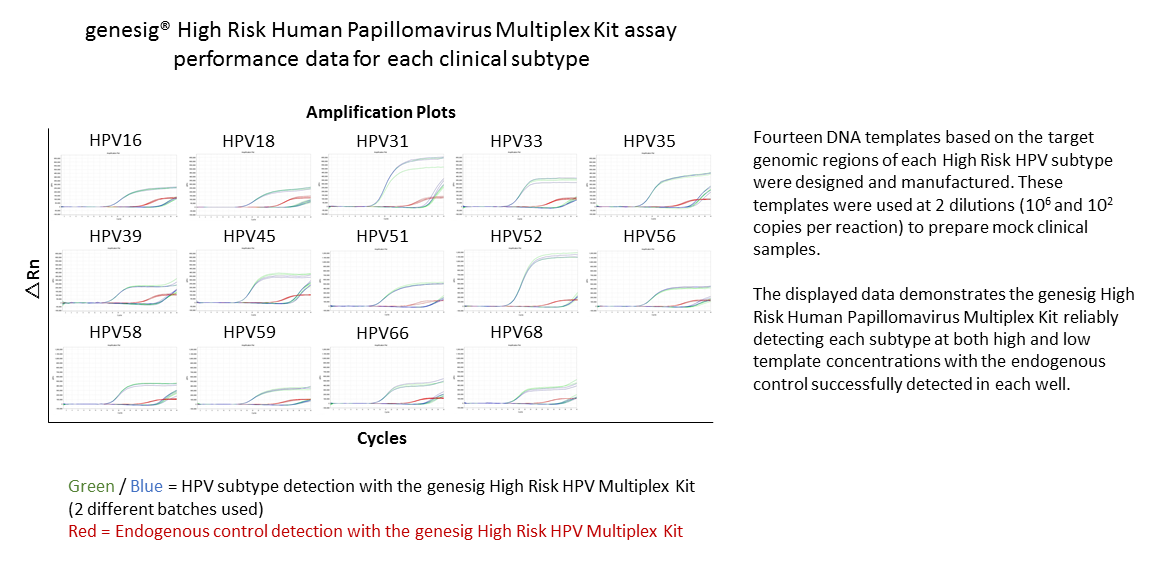
This kit provides a single tube to screen for the presence of high-risk HPV types, HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, HPV66 and HPV68. The multiplex test is detected in two fluorescent channels differentiating between HPV16 / HPV18 which both produce a VIC channel signal and all others which produce a signal in the FAM channel. HPV16 and HPV18 account for 70% of positive findings in clinical practice so it is helpful to know if either of these are present. All other high risk genotypes together make up the remaining 30% of clinical positives and are grouped together into the FAM channel. In this configuration, the kit gives a partial genotyping result and some additional information on which high risk strains are present

Product features
genesig® kits are sold for research use only and are not licensed for diagnostic procedures.
High Risk HPV primer/probe mix
High Risk HPV positive control template
Positive signal for FAM and VIC channel
Oasig MasterMix
Oasig resuspension buffer
RNase/DNase free water
High-Risk HPV Multiplex
The HPV16 and HPV18 copy numbers were quantified using a qRT-PCR method with the genesig Standard Kits (Primerdesign Ltd.) as described above. The test amplifies the region that codes HR-HPV E6 oncoprotein. High risk Human Papillomavirus Multiplex screening genesig kit (Primerdesign Ltd.) was used to simultaneously detection of DNA of 14 HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). The DNA was quantified using the LightCycler 96 System (Roche Diagnostics GmbH, Mannheim, Germany).
Paradowska E, Jabłońska A, Studzińska M, Wilczyński M, Wilczyński JR. Detection and genotyping of CMV and HPV in tumors and fallopian tubes from epithelial ovarian cancer patients. Sci Rep. 2019 Dec 27;9(1):19935. doi: 10.1038/s41598-019-56448-1. PMID: 31882737; PMCID: PMC6934444.
Individual kits (HPV16, HPV18, HPV31, HPV33, HPV52 and HPV52b kits)
The qRT-PCR was carried out using the Genesig Advanced Kit (Genesig, England) following the manufacturer’s protocol. Briefly, the assay is an in vitro real-time amplification test for qualitative and quantitative detection of HR-HPV genotypes. Three HR-HPV types 16, 18, and 31 were investigated.
El-Sheikh N, Mousa NO, Tawfeik AM, Saleh AM, Elshikh I, Deyab M, Ragheb F, Moneer MM, Kawashti A, Osman A, Elrefaei M. Assessment of Human Papillomavirus Infection and Risk Factors in Egyptian Women With Breast Cancer. Breast Cancer (Auckl). 2021 Feb 25;15:1178223421996279. doi: 10.1177/1178223421996279. PMID: 33716506; PMCID: PMC7917427.
DNA of HR-HPV was detected by quantitative PCR (qPCR) using probe directed to the HPV types 16 and 18 were detected with The Primer design genesig Kit for human papillomavirus 16 y 18 (HPV16 and HPV18).
Calderon G, Castaneda CA, Castillo M, Sanchez J, Bernabe L, Suarez N, Tello K, Torres E, Cotrina JM, Dunstan J, De-La-Cruz M, Abugattas J, Guerra H, Manrique JE, Aguayo F, Gomez HL. Human Papillomavirus, Cytomegalovirus Infection and P16 Staining in Breast Tumors from Peruvian Women. Asian Pac J Cancer Prev. 2022 May 1;23(5):1571-1576. doi: 10.31557/APJCP.2022.23.5.1571. PMID: 35633540; PMCID: PMC9587888.
The real time PCR method includes the detection of E6 gene/HPV for the types 16, 18, 33 and 52 and 52 b, by qualitative end point PCR method, with the Primerdesign™ genesig® kit for HPV TaqMan® principle.
Salceanu SO, Constantin C, Cijevschi I, Ursu RG, Grigorovici M, Iancu LS. Human papillomavirus 52 positive squamous cell carcinoma of the conjunctiva. Indian J Ophthalmol. 2015 Feb;63(2):166-9. doi: 10.4103/0301-4738.154406. PMID: 25827551; PMCID: PMC4399129.
Human papillomavirus 52 positive squamous cell carcinoma of the conjunctiva - PMC (nih.gov)
Absolute quantification of HPV 16 and 18 was performed with Path-HPV16/18 Real-time PCR detection kit for Human Papillomavirus, 2 x Precision Mastermix kits (PrimerDesign), and the instrument used was MX3000P STRATAGENE.
Ursu RG, Onofriescu M, Nemescu D, Iancu LS. Optimizarea unor tehnici de biologie moleculară pentru diagnosticul infecţiilor cu virusuri papilloma umane [Enhanced molecular techniques for the diagnosis of human papillomavirus infections]. Rev Med Chir Soc Med Nat Iasi. 2009 Oct-Dec;113(4):1205-10. Romanian. PMID: 20191900.
The genesig® advanced kit introduced by Primerdesign™ Ltd., Co., Southampton, UK, was used for both detection and genotyping. This kit relies on the TaqMan® method and has a primer and probe mix that hybridizes specifically with HPV 16 and/or 18 E6 oncogene.
Alzaquzi, Hani1,4,; Almaghur, Lubna2; Eshagrouni, Ahmed3; Elahmer, Omar4; Bashein, Abdulla4,5. Prevalence of High-Risk Human Papillomavirus Types 16 and 18 among Libyan Women in Tripoli Libya. Libyan Journal of Medical Sciences 3(4):p 125-130, Oct–Dec 2019. | DOI: 10.4103/LJMS.LJMS_44_19
A commercially available HVP33 detection system (PrimerDesign™ genesig HVP33 Kt, Southampton, UK) was used to analyse HPV33 E6 gene expression. Both HPV18 and HPV33 expression analysis was conducted on the Applied Biosystems 7500 FAST system.
Schache AG, Liloglou T, Risk JM, Filia A, Jones TM, Sheard J, Woolgar JA, Helliwell TR, Triantafyllou A, Robinson M, Sloan P, Harvey-Woodworth C, Sisson D, Shaw RJ. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011 Oct 1;17(19):6262-71. doi: 10.1158/1078-0432.CCR-11-0388. PMID: 21969383; PMCID: PMC3188400.
HBV and HPV-31 were tested with commercial kits (Hepatitis B Virus PCR Kit, GeneProof, Genesig; and Human papillomavirus 31 Standard kit, PrimerDesign, respectively) according to the manufacturer protocol.
Toppinen M, Sajantila A, Pratas D, Hedman K, Perdomo MF. The Human Bone Marrow Is Host to the DNAs of Several Viruses. Front Cell Infect Microbiol. 2021 Apr 22;11:657245. doi: 10.3389/fcimb.2021.657245. PMID: 33968803; PMCID: PMC8100435.
The Human Bone Marrow Is Host to the DNAs of Several Viruses - PMC (nih.gov)
The quantification of hepatitis B virus and human papillomavirus type 31 DNAs were performed with commercial kits (Hepatitis B Virus PCR Kit, GeneProof; Genesig Human papillomavirus 31 Standard kit, PrimerDesign) according to the manufacturer instructions.
Toppinen M, Pratas D, Väisänen E, Söderlund-Venermo M, Hedman K, Perdomo MF, Sajantila A. The landscape of persistent human DNA viruses in femoral bone. Forensic Sci Int Genet. 2020 Sep;48:102353. doi: 10.1016/j.fsigen.2020.102353. Epub 2020 Jul 8. PMID: 32668397.
The landscape of persistent human DNA viruses in femoral bone - PubMed (nih.gov)
Ask our friendly experts your question and we'll respond within one business day.